Tannin / tannic acid
Properties
Pure tannic acid is a fine, yellow to yellow-brownish powder, rarely flakes or needles. Tannin is easily soluble in water, soluble in ethanol, acetone and warm glycerine. Tannic acid is practically insoluble in non-polar organic solvents such as diethyl ether, chloroform and benzene. The aqueous solution of tannin turns the plane of polarised light to the right (positive rotation value). The addition of iron(III) salts to aqueous tannic acid solutions leads to blue-black colourations or precipitates (“iron gall inks”).
The odour is faint but typical, the taste astringent (“astringent”).
Extraction
Collected plant galls are crushed and extracted with water. The aqueous extract is then extracted with a mixture of diethyl ether/ethanol (4:1); the organic phase is separated and evaporated to dryness.
Structure
The basic structure of tannin is a D-glucose molecule, which is esterified at all 5 hydroxyl functions with gallic acid or galloylgallic acid residues:
| R = | resp: | |
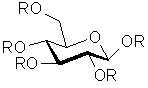 |
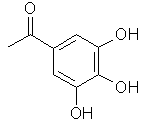 |
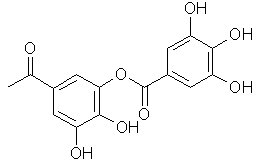 |
| Esterified D-glucose | Gallic acid residue | m-Galloylgallic acid residue |
Normally between 1 and 2 hydroxyl functions are esterified with gallic acid and between 3 and 4 with galloylgallic acid.
Possible molecular formulae from C41H32O26 (MW: 940.68) to C76H52O46 (MW: 1701.20)

