Data
| CO2 liquid and gaseous | Values |
| Molar mass (MCO2) | 44.011 kg/kmol |
| Molar standard volume (Vmn) | 22.263 m3/kmol |
| Specific gas constant (RCO2) | 0.1889 kJ/(kg · K) |
| Standard density (ρn) | 1.977 kg/m3 |
| Density ratio CO2/air(d) | 1,529 |
| Critical temperature (Tkrit) | 31 °C |
| Critical pressure (pkrit) | 73.83 bar |
| Critical density (ρkrit) | 466 kg/m3 |
| Triple point (tT) | -56.6 °C at 5.18 bar |
| Decomposition temperature | approx. 1,200 °C (degree of decomposition: 0.032 % by volume) |
| Colour in the gaseous state | colourless |
| Fire behaviour | non-flammable, use as extinguishing agent |
| Combination with water | CO2 + H2O |
| Odour | odourless |
| Flavour | neutral |
| Toxicity | Non-toxic. Authorised under food law and declaration-free |
| MAK value | 5,000 ml/m3 (ppm) as 8-hour average value |
| Medical use | Inhalations of 3 - 5 % CO2 in the breathing gas |
| Effects of different concentrations | Irritation of the respiratory centre at 3 - 5 % by volume Unconsciousness at 7 - 10 % by volume due to lack of oxygen |
| CO2 solid | |
| Sublimation point | ts = -78.9 °C at 0.981 bar |
| Heat of sublimation | rs = 573.02 kJ/kg |
| Cooling capacity during heating | q0 ˜ 645 kJ/kg (from -78.9 °C [194.25 K] to 0 °C [273.15 K]) |
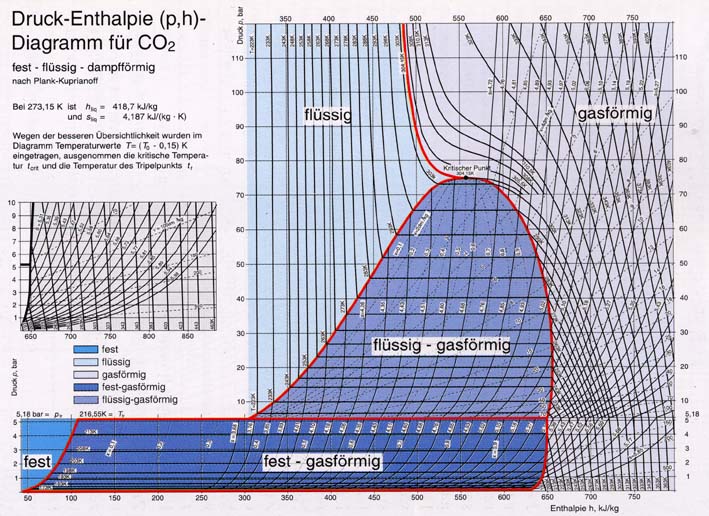
Diagrams:
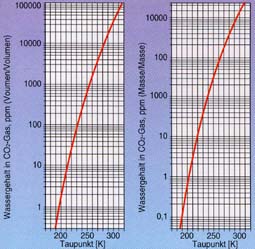 |
Water content in CO2 gas as a function of the dew point temperature |
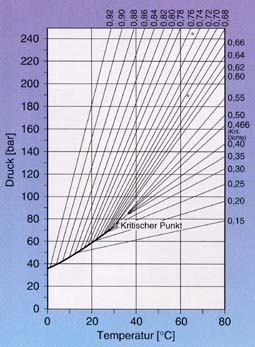 |
Isochores for CO2 (lines of equal density) |