Chemistry
CO2 is used in the beverage industry, but also to neutralise alkaline waste water. As the resulting carbonates are strong buffers and the carbonic acid is only a weak acid, largely neutral waste water is produced without the need for complex controls. Due to its non-toxicity and environmental friendliness, CO2 is therefore well suited for such tasks.
According to the following reaction equation, carbonic acid is formed from water and CO2:
CO2 + H2O ![]() H2CO3
H2CO3
However, 99.9 % of the equilibrium is on the left-hand side of the equation. The rest of the CO2 is physically dissolved in the water. Therefore, the pH value of water solutions containing CO2 can drop to a maximum of 3.7 (normal pressure) or 3.3 (limit value under pressure).
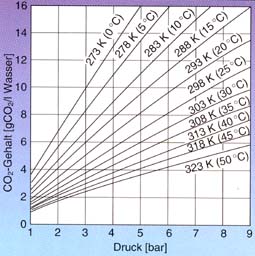
Solubility
The important property for the aquarist is the easy solubility in water. It is highly dependent on temperature and pressure. At low temperature and high pressure, more CO2 dissolves in water.
Other substances dissolved in the water such as salts, sugar, dextrin and starch reduce the solubility, while iron hydroxides and gelatine can increase it (order of magnitude +5 to -10 %).
The supercritical state
A gas located above the critical point in the phase diagram is in the so-called supercritical state (for CO2: T = 31 °C, p = 74 bar). CO2 in this state has excellent solvent properties. These are very selective and change greatly with small variations in the parameters. This means that a substance can first be extracted with very little energy input and then separated again after a slight change in the parameters.
Technically, this is used, for example, in the decaffeination of coffee or tea, the reduction of nicotine in tobacco for light brands and the production of plant extracts from hops and other spices.

